EMERGENZA COVID - 19
In questo periodo viene privilegiata la TELEMEDICINA.
Per consultazioni in Videocall con il Prof. Giovanni M. Colpi, evitando viaggi e relative spese, inviare la richiesta a This email address is being protected from spambots. You need JavaScript enabled to view it., insieme con tutti gli esami finora eseguiti.
La Segreteria di Procrea provvederà a fissare l’appuntamento.
 Lecture* at the XI. Eurasian Andrology Summit & 18th European Society of Sexual Medicine Joint Meeting, 4-6 February, 2016 - Madrid
Lecture* at the XI. Eurasian Andrology Summit & 18th European Society of Sexual Medicine Joint Meeting, 4-6 February, 2016 - Madrid
Giovanni M. COLPI1, 2, Liborio VACCALLUZZO1, Elisabetta M. COLPI2
1ISES - Istituto per la Sterilità e la Sessualità, Milano (Italy)
2ProCrea, Lugano (Switzerland)
*Text published in the Proceedings
INTRODUCTION
Non-Obstructive Azoospermia (NOA) is managed by retrieval of testicular sperm to be used for ICSI. TeFNA (Testicular Fine Needle Aspiration) and TESA (TEsticular Sperm Aspiration), uncorrectly still used in many IVF (In-Vitro Fertilization) Centers, are now virtually ignored by the EAU (European Association for Urology) Guidelines,
which report TESE (Testicular Sperm Extraction) as the recommended procedure in NOA (Jungwirth, 2015). In recent years, MicroTESE, ideated by Schlegel (1999), is raising to the role of most appropriate testicular sperm retrieval technique in NOA, owing to its better Sperm Retrieval Rate (SRR). Achieving this positive statement has been difficult, because MicroTESE requires microsurgical skills, uncommon among urologists. However, nowadays recourse to MicroTESE is increasing exponentially: not rarely for marketing reasons, since, as seen in many Internet videos, some operative details shown by some surgeons are far from the best.
TESE versus MicroTESE
In NOA, TESE can have an acceptable SRR, depending on the number of samples (one: 44.5%; two: 51.4%; three: 56.1%; four: 58.4%)(Dadkhah, 2013): incisions, randomly performed with naked eye, can sever subalbugineal or lobular vessels, devascularizing testis lobules and increasing the risk of subsequent hypogonadism (Schlegel, 1997). MicroTESE, with expert's eyes, allows the identification of individual clumps of tubules (Silber, 2000) with better writing a essay spermatogenesis, so maximizing sperm recovery, and the excision of single tubules, so providing the biologist with less testicular tissue to dissect in order to find sperms: in addition, the best visualization of the vessels allows optimal preservation of blood supply to the testis (Schlegel, 1999). Comparison between MicroTESE and TESE results showed higher SRRs with MicroTESE (42.9-63% vs 16.7-45%) in a metanalysis (Deruyver, 2013); a recent larger metanalysis (1890 pts.) by Bernie (2015) confirmed that MicroTESE SRR is 1.5 higher than TESE SRR (the latter being twice higher than TESA one). Superiority of MicroTESE SRRs was proven even in the different histopathological NOA patterns: 37% vs 14% in SCOS (Sertoli Cell Only Syndrome), 49% vs 27% in Maturation Arrest; 85% vs 73% in Hypospermatogenesis (Deruyver, 2013). High MicroTESE SRRs were reported, too, in complicate NOA cases: e.g. due to chemotherapy (48%), previous cryptorchidism (64%), AZFc microdeletions (67%)(Dabaja, 2013), Klinefelter syndrome (Sabbaghian, 2014); and as a salvage procedure after TESE failure (45-46.5%)(Tsujimura, 2006; Ramasamy, 2007; Kalsi, 2014).
SURGICAL TECHNIQUE and our improvements
By an incision in a poorly vascularized area, the testis is opened, 270° or more, along an equatorial or para-equatorial plane, gently and progressively, avoiding any parenchymal stretching. Following the distribution of the intratesticular vessels inside the septa (5 to 15x magnification), a vast exposition of the seminiferous tubules is achieved. Respecting blood supply of lobules, and avoiding any rough disconnection of tunica albuginea (rich of easily bleeding vessels) are mandatory. A careful and fine microdissection allows to access to the deeper tubules of a lobule, and to
some of more internal lobules. Operating with a microscope at 15-24x magnification (36x when needed) allows identification of any more opaque and larger tubules, those probably hosting mature spermatogenetic cells. In many NOA testes, foci with residual spermatogenesis are heterogeneously distributed; therefore, microdissection must be extremely exploratory. In difficult cases, when no larger tubules are visible on the two surfaces of the shell-like opened testis, we insinuate our Vannas forceps more deeply along the septa, delicately detaching many lobules from the surrounding ones, almost reaching the opposite tunica albuginea, thoroughly probing the whole underlying parenchyma. The larger, or apparently larger, tubules are retrieved and passed to the biologist assisting the operation, for an immediate meticulous sperm search. At the end, testicular tissue is irrigated with Ringer solution plus gentamycin. Haemostasis is mainly performed by gentle pressure on the parenchyma for 3-4' using a gauze wet with antibiotic and, only if strictly necessary, using the bipolar thermal device (0.2 mm tip). Albugineal incision is repaired with continuous suture (VicrylTM 4-0 or 5-0), ideally limited to the external half of its thickness, then followed by closure of tunica vaginalis, infusion of betamethasone solution into vaginal cavity to prevent pain and adhesions, and closure of dartos and skin. If surgery has been made meticulously, post-operative progress is actually painless, and any scar will become invisible at sonography three months later (Colpi, 2010).
RESULTS
Success depends maximally on the microsurgeon's experience, because MicroTESE has a very long learning curve, with satisfying SRR beyond the first 100 cases (Ishikawa, 2010); Schlegel claims that SRR goes on improving very slowly until overstepping a 500 case-threshold, because eyes succeed in seizing even minimal differences in tubular diameter (Dabaja, 2013). In a recent prospective study in progress on 45 NOA patients, where in total we operated on 69 testes by MicroTESE (testis volume was 10-15 ml in 10 testes, 7-10 ml in 39, and <7 ml in 20; subsequent hystology was complete SCOS in 50 testes, incomplete SCOS in 5, maturation arrest in 11, hypospermatogenesis in 2, intratubular neoplasia - CIS in 1) with a positive result in 27 patients [SRR 60%], we classified tubules into "dilated", "apparently dilated" (at 24x), and "not-dilated": we had a positive retrieval respectively in 19/21 testes with "dilated", in 7/19 testes with "apparently dilated", and in 2/29 testes with "non-dilated" tubules. We had a successful retrieval in 16 out of 50 testes with complete SCOS (> 32%).
WEAK POINTS AND COMPLICATIONS
Surgery duration of MicroTESE is much longer than TESE: 1.8 h (range: 0.5-6.6 h) in case of positive retrievals and 2.7 h (range 0.8-7.5 h) in case of negative retrievals (Ramasamy, 2011), therefore requiring usually general anesthesia and sometimes an overnight stay in hospital. In our series of 740 MicroTESE, the mean operative time for monolateral successful MicroTESE was 86.9' (range: 60-140'), and for bilateral successful or unsuccessful MicroTESE was 125.8' (range: 85-200'). Compared to multiple TESE, which is believed to be more dangerous for testis, due to possible blood supply lesions (Schlegel, 1997) or intratesticular edema or hematoma (Silber, 2000), MicroTESE is now considered the safer procedure, with a lower incidence of bleeding (Schlegel, 1999; Okada, 2002) and postoperative complications such as hematomas, fibrosis and reduced orchidometry, as assessed by sonography (Amer, 2000; Okada, 2002; Ramasamy, 2005; Deruyver, 2013). In our series of 740 MicroTESE, only one hematoma occurred (due to hemostasis during hypotensive state, not communicated by anesthesiologist, and successfully treated without any subsequent damage); no infection, no post-surgical testis hypotrophy or pain, not even in cases where we performed two MicroTESE on the same testis to increase parenchyma exploration. Furthermore, no differences versus TESE were shown about possible post-operative hypotestosteronemia (Okada, 2002; Ramasamy, 2005; Deruyver, 2013).
RELEVANT CONSIDERATIONS ABOUT SPERM RETRIEVAL IN NOA
MicroTESE seems particularly helpful for the worst-case NOA scenarios (Ashraf, 2013; Esteves, 2015), and especially with high FSH (Ramasamy, 2009; Modarresi, 2015). Hormonal presurgical treatment is under debate. There is some evidence that a small percentage of men with azoospermia due to testicular failure may benefit from treatment of a clinical varicocele (Colpi, 2005; Inci, 2009; Weedin, 2010). In many NOA patients, an increased risk of CIS (carcinoma in situ) has been described (Jungwirth, 2015): therefore, a tissue fragment, appropriately fixed in Bouin's solution, must be always read by an experienced pathologist (Dohle, 2012). About half of NOA patients are hypogonadic, and require endocrine post-surgical follow-up, and testosterone replacement when needed (Bobjer, 2012). The biological search for sperm in the minced tubules requires a well-trained biologist working in the theatre; an additional +7% success rate is reported when removed specimen exam is followed by a multi-hour, many-technician search in an experienced laboratory (Ramasamy, 2011). The laboratory management of these specimens requires special attention, because NOA spermatozoa are often compromised in quality and more fragile than ejaculated ones (Esteves, 2015). When only immotile spermatozoa are obtained after processing of fresh or cryo-thawed specimens, different methods (HOS test, sperm tail flexibility test, and pentoxifylline stimulation) can be used to differentiate live immotile from dead spermatozoa (Esteves, 2012). Vitrification seems to be a suitable method to cryopreserve sperms from poor positive retrievals (Endo, 2012). ICSI performed using fresh or cryo-thawed sperm seems to achieve similar results, whilst live birth rates are significantly lower in the NOA (21.4% per ET/cycle^) compared with OA (37.5% per ET/cycle) patients (He, 2010; Esteves, 2013).
"The testicular sperm extraction procedure should be offered to all men with NOA, but should only be undertaken in a Centre with expertise in MicroTESE and where an ICSI Laboratory with expertise in handling these samples is available" according to the Canadian Guidelines (Jarvi, 2015).
(^P.S. 31,5% in our latest series)
Fig. 1: Sometimes dilated tubules can be easily identified, even at lower magnification.
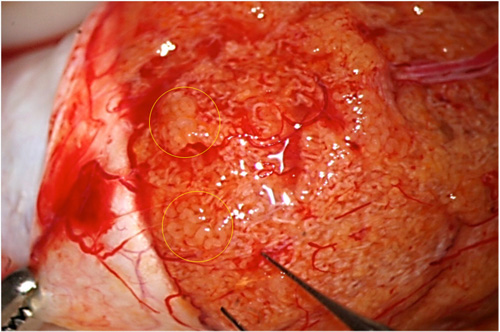
Fig. 2: Sometimes higher magnification allows better identification of dilated tubules.
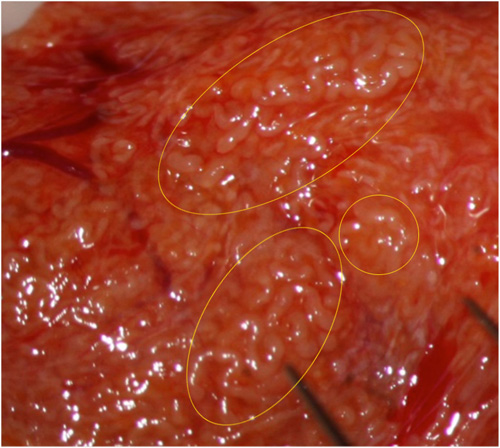
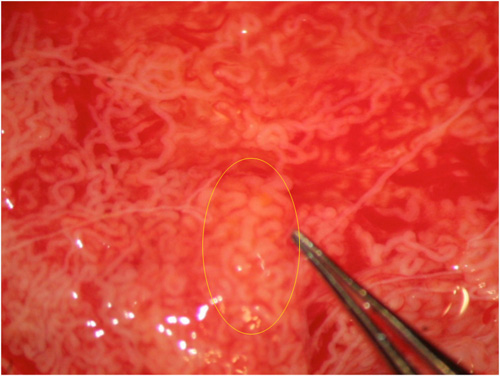
Fig. 3: Other times tubular dilation can be identified only at higher magnification.
 REFERENCES
REFERENCES
Jungwirth A, Diemer T, Dohle GR, Giwercman A, Kopa Z, Krausz C, Tournaye H. Guidelines of Male Infertility. EAU Guidelines, 2014, p 9.
Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod 1999; 14(1): 131-135.
Dadkhah F, Hosseini SJ, Sadighi Gilani MA, Farrahi F, Amini E, Kazeminejad B. Optimal number of biopsies and impact of testicular histology on the outcome of testicular sperm extraction. Urol J 2013; 10: 795-801.
Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Hum Reprod 1997; 12: 1688-1692.
Silber SJ. Microsurgical TESE and the distribution of spermatogenesis in non‑obstructive azoospermia. Hum Reprod 2000; 15: 2278-2284.
Deruyver Y, Vanderschueren D, Van der Aa F. Outcome of microdissection TESE compared with conventional TESE in non‑obstructive azoospermia: a systematic review. Andrology 2014; 2: 20-24.
Bernie AM, Mata DA, Ramasamy R, Schlegel PN. Comparison of microdissection testicular sperm extraction, conventional testicular sperm extraction, and testicular sperm aspiration for nonobstructive azoospermia: a systematic review and meta-analysis. Fertil Steril 2015; 104:1099-1103.
Dabaja AA, Schlegel PN. Microdissection testicular sperm extraction: an update. Asian J Androl 2012; 15: 35-39.
Sabbaghian M, Modarresi T, Hosseinifar H, Hosseini J, Farrahi F, Dadkhah F, Chehrazi M, Khalili G, Sadighi Gilani MA. Comparison of sperm retrieval and intracytoplasmic sperm injection outcome in patients with and without Klinefelter syndrome. Urology 2014; 83:107-110.
Tsujimura A, Miyagawa Y, Takao T, Takada S, Koga M, Takeyama M, Matsumiya K, Fujioka H, Okuyama A. Salvage microdissection testicular sperm extraction after failed conventional testicular sperm extraction in patients with nonobstructive azoospermia. J Urol 2006; 175:1446-9; discussion 1449.
Ramasamy R1, Schlegel PN. Microdissection testicular sperm extraction: effect of prior biopsy on success of sperm retrieval. J Urol 2007;177:1447-1449.
Kalsi JS1, Shah P, Thum Y, Muneer A, Ralph DJ, Minhas S. Salvage micro-dissection testicular sperm extraction; outcome in men with non-obstructive azoospermia with previous failed sperm retrievals. BJU Int 2015; 116: 460-465.
Colpi GM, Piediferro G, Scroppo FI, Colpi EM, Sulpizio P: Surgery for male infertility: Surgical sperm retrievals. In Bjorndahl L, Giwercman A, Tournaye H, and Weidner W (eds), Clinical Andrology, London, Informa Healthcare, pp 95-104.
Ishikawa T, Nose R, Yamaguchi K, Chiba K, Fujisawa M. Learning curves of microdissection testicular sperm extraction for nonobstructive azoospermia. Fertil Steril. 2010; 94: 1008-1011.
Ramasamy R, Fisher ES, Ricci JA, Leung RA, Schlegel PN. Duration of microdissection testicular sperm extraction procedures: relationship to sperm retrieval success. J Urol. 2011; 185: 1394-1397.
Okada H, Dobashi M, Yamazaki T, Hara I, Fujisawa M, Arakawa S, Kamidono S. Conventional versus microdissection testicular sperm extraction for nonobstructive azoospermia. J Urol. 2002; 168: 1063-1067.
Amer M, Ateyah A, Hany R, Zohdy W. Prospective comparative study between microsurgical and conventional testicular sperm extraction in non-obstructive azoospermia: follow-up by serial ultrasound examinations. Hum Reprod 2000; 15: 653-656.
Ramasamy R, Yagan N, Schlegel PN. Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology 2005; 65: 1190-1194.
Ashraf MC, Singh S, Raj D, Ramakrishnan S, Esteves SC. Micro‑dissection testicular sperm extraction as an alternative for sperm acquisition in the most difficult cases of azoospermia: technique and preliminary results in India. J Hum Reprod Sci 2013; 6: 111-123.
Esteves SC. Clinical management of infertile men with nonobstructive azoospermia. Asian J Androl 2015; 17: 459-470.
Ramasamy R, Lin K, Gosden LV, Rosenwaks Z, Palermo GD, Schlegel PN. High serum FSH levels in men with nonobstructive azoospermia does not affect success of microdissection testicular sperm extraction. Fertil Steril 2009; 92: 590-593.
Modarresi T, Hosseinifar H, Daliri Hampa A, Chehrazi M, Hosseini J, Farrahi F, Dadkhah F, Sabbaghian M, Sadighi Gilani MA. Predictive factors of successful microdissection testicular sperm extraction in patients with presumed Sertoli cell-only syndrome. Int J Fertil Steril 2015; 9: 107-112.
Colpi GM, Piediferro G, Nerva F, Giacchetta D, Colpi EM, Piatti E. Sperm retrieval for intra-cytoplasmic sperm injection in non-obstructive azoospermia. Minerva Urol Nefrol 2005; 57: 99-107.
Inci K, Hascicek M, Kara O, Dikmen AV, Gürgan T, Ergen A. Sperm retrieval and intracytoplasmic sperm injection in men with nonobstructive azoospermia, and treated and untreated varicocele. J Urol 2009; 182: 1500-1505.
Weedin JW, Khera M, Lipshultz LI. Varicocele repair in patients with nonobstructive azoospermia: a meta‑analysis. J Urol 2010; 183: 2309-2015.
Dohle GR, Elzanaty S, van Casteren NJ. Testicular biopsy: clinical practice and interpretation. Asian J Androl 2012; 14: 88-93.
Bobjer J, Naumovska M, Giwercman YL, Giwercman A. High prevalence of androgen deficiency and abnormal lipid profile in infertile men with non-obstructive azoospermia. Int J Androl 2012; 35: 688-694.
Ramasamy R, Reifsnyder JE, Bryson C, Zaninovic N, Liotta D, Cook CA, Hariprashad J, Weiss D, Neri Q, Palermo GD, Schlegel PN. Role of tissue digestion and extensive sperm search after microdissection testicular sperm extraction. Fertil Steril 2011; 96: 299-302.
Esteves SC, Varghese AC. Laboratory handling of epididymal and testicular spermatozoa: what can be done to improve sperm injections outcome. J Hum Reprod Sci 2012; 5: 233-43.
Endo Y, Fujii Y, Shintani K, Seo M, Motoyama H, Funahashi H. Simple vitrification for small numbers of human spermatozoa. Reprod Biomed Online 2012; 24: 301-307.
He X, Cao Y, Zhang Z, Zhao J, Wei Z, Zhou P, Cong L. Spermatogenesis affects the outcome of ICSI for azoospermic patients rather than sperm retrieval method. Syst Biol Reprod Med 2010; 56: 457-464.
Esteves SC, Agarwal A. Reproductive outcomes, including neonatal data, following sperm injection in men with obstructive and nonobstructive azoospermia: case series and systematic review. Clinics (Sao Paulo) 2013; 68 Suppl 1: 141-150.
Jarvi K, Lo K, Grober E, Mak V, Fischer A, Grantmyre J, Zini A, Chan P, Patry G, Chow V, Domes T. CUA Guideline: The workup and management of azoospermic males. Can Urol Assoc J 2015; 9:229-235.
